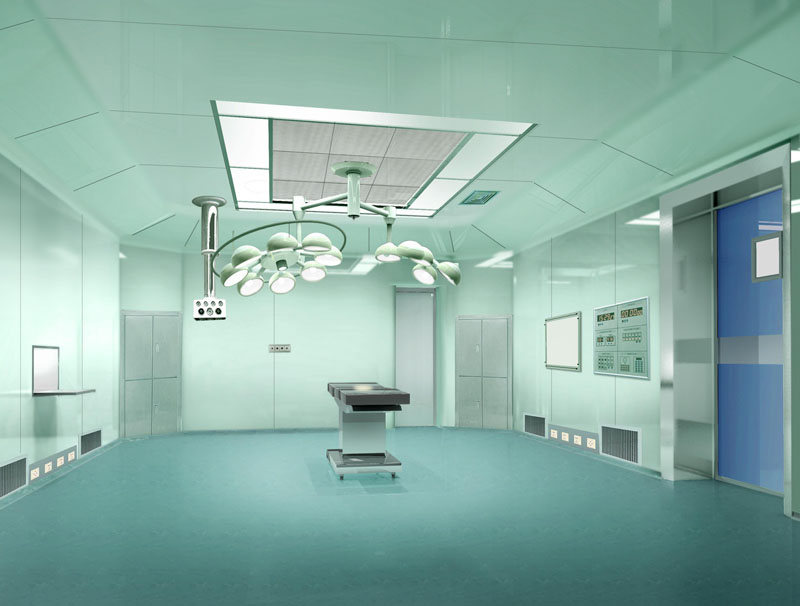
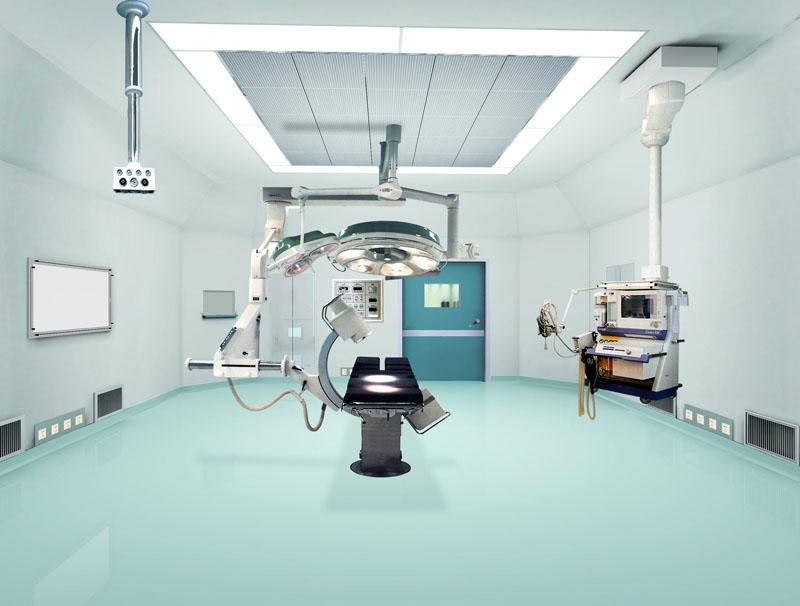
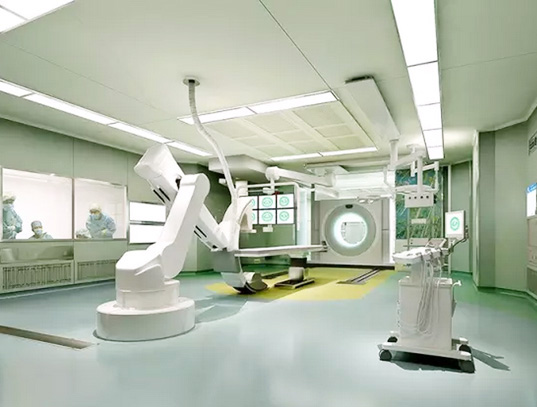
Biopharmaceutical Plant Features:
1, biopharmaceutical factory not only the high cost of equipment, the production process is complex, clean level and the requirements of aseptic, but also the quality of production staff have strict requirements.
2, in the production process there will be potential biological hazards, mainly (infection risk, dead cells or dead cells and components or metabolism on the human body and other biological toxic, allergenic and other biological reactions, product toxicity , Allergenicity and other biological reactions, environmental effects.)
Clean Area:
The room (area), which requires control of dust and microbial contamination in the environment, has the function of preventing the introduction, generation and retention of contaminants in the area by its building structure, equipment and its use.
Air Lock:
A compartment that has two or more doors separated between two or several rooms, such as between rooms of different cleanliness levels. The purpose of setting up the air lock is to control the airflow when the person or material enters and leaves. There is a lock between the air lock and the gas lock between the points.
The basic characteristics of biopharmaceutical cleansing plants are that they must be controlled by dust particles and microbes for the environment.
Drug production workshop cleanliness is divided into four levels: 100 or 10000 level of the local 100, 1000, 10000 and 30,000.
Clean room temperature: no special requirements, at 18 to 26 degrees, relative humidity control in 45% to 65%.
Biopharmaceutical Clean Plant Pollution Control: Pollution Source Control, Spreading Process Control, Cross Pollution Control.
Pharmaceutical plant clean room key technology is mainly to control dust and microbes, as a pollutant, microbial is the pharmaceutical plant clean room environmental control of the most important. Pharmaceutical plant clean area equipment, pipeline accumulation of pollutants, can directly pollute the drug, but without affecting the cleanliness of the test, so we said: GMP need air purification technology, and air purification technology does not mean GMP! The cleanliness level is not suitable for characterizing the physical, chemical, radioactive and vitality of suspended particles. Not familiar with the pharmaceutical production process and process, do not understand the causes of pollution and the accumulation of pollutants in the place, do not grasp the method of removing pollutants and evaluation criteria.
GMP technical transformation of the pharmaceutical plant project prevalence of the following:
Because of the existence of the subjective understanding of the errors in the pollution control process of the application of clean technology adverse, and ultimately appeared in some pharmaceutical companies invested heavily in transformation, the quality of drugs has not improved significantly.
The design and construction of pharmaceutical clean production plant, the equipment manufacturing, installation and production of raw and auxiliary materials, the quality of packaging materials, the implementation of the net equipment control procedures will affect the implementation of adverse product quality.
Construction of the impact of product quality is due to process control links in the process of installation and construction of hidden risks, the following specific performance:
① clean air conditioning system duct is not clean, the connection is not tight, air leakage rate is too large;
② color steel plate enclosure structure is not tight, clean room and technology sandwich (ceiling) of the improper sealing measures, closed doors do not close;
③ decorative profiles and process pipelines in the clean room to form a dead, dust;
④ individual location is not in accordance with the design requirements of construction, can not meet the relevant requirements;
⑤ the quality of the sealant used, but off, easy to fall off, deterioration;
⑥ back, exhaust color steel plate lane connected, dust from the exhaust to enter the air duct;
⑦ process purified water, water injection and other stainless steel sanitary pipe welding when the wall weld is not formed;
⑧ air duct check valve failure, air pollution caused by pollution;
⑨ drainage system installation quality, but off, pipe rack, accessories easy to dust;
⑩ clean room pressure adjustment failed, failed to meet the production process requirements.
GMP to find drug packaging workshop purification project environmental control requirements:
1, to provide the required level of production of air purification, packaging workshop purification works within the number of dust particles and live microorganisms should be regularly detected and recorded, different levels of packaging between the static pressure difference between the workshop should be maintained within the specified value;
2, packaging workshop purification project temperature and relative humidity should be compatible with its production process requirements;
3, penicillins, highly allergenic and anti-tumor drugs in the production area should be set up an independent air conditioning system, exhaust to clean up;
4, for the dusty room should be set up an effective dust collector to prevent dust pollution;
5, the storage and other auxiliary production room, its ventilation facilities and temperature and humidity should be compatible with the requirements of pharmaceutical production and packaging.
Second, to find drug packaging workshop purification project> cleanliness of the partition and the number of ventilation: clean room should strictly control the air cleanliness, and environmental temperature, humidity, fresh air and pressure and other parameters.
1, the pharmaceutical production and packaging workshop purification level and the number of ventilation and pharmaceutical production and packaging workshop purification works air cleanliness is divided into 100,
1 million, 100,000, 300,000 4 level. Determine the number of clean room ventilation, the need for the air volume to compare, take the maximum. In practice, 100 times the number of ventilation 300 to 400 times / h, 10,000 to 25-35 times / h, 100,000 to 15-20 times / h.
2, drug packaging workshop purification project cleanliness of the district drug production and packaging environment on the specific degree of cleanliness by the national standard cleanliness standards.
3, packaging workshop purification project to determine the other environmental parameters
4, packaging workshop purification project temperature and humidity clean room temperature and relative humidity should be consistent with the pharmaceutical production process.
Temperature: 100 and 10,000 to take 20 ~ 23 ~ C (summer),
100,000 and 300,000 to take 24 ~ 26 ~ C, the general area of 26 ~ 27 ~ C.
100 and 10,000 are sterile rooms. Relative humidity: easy to absorb moisture drugs 45% to 50% (summer), tablets and other solid preparations 50% to 55%, water and oral liquid 55% to 65%.
5, clean room pressure to maintain indoor cleanliness to maintain the indoor positive pressure. For the production of dust, harmful substances, the production of penicillin-like strong allergenic drugs such as the production of clean room to prevent external pollution or between the region to maintain a relatively negative pressure. Cleanliness level of inflow of static pressure in different rooms and outflow of internal air. Indoor to maintain a positive pressure, and adjacent rooms worse than 5Pa, clean room and outdoor air pressure difference is greater than 10Pa.
Dongguan Zhuo for the air-conditioning Electrical and Mechanical Equipment Co., Ltd. is specialized in the Pearl River Delta region clean clean workshop, the central air-conditioning engineering design and construction services of professional purification engineering company. Zhuo for purification in the electronics manufacturing, bio-pharmaceutical, printing and packaging, food, chemical optics, health care and other industries in the design and construction of numerous classic works, access to a high degree of customer recognition.
1, biopharmaceutical factory not only the high cost of equipment, the production process is complex, clean level and the requirements of aseptic, but also the quality of production staff have strict requirements.
2, in the production process there will be potential biological hazards, mainly (infection risk, dead cells or dead cells and components or metabolism on the human body and other biological toxic, allergenic and other biological reactions, product toxicity , Allergenicity and other biological reactions, environmental effects.)
Clean Area:
The room (area), which requires control of dust and microbial contamination in the environment, has the function of preventing the introduction, generation and retention of contaminants in the area by its building structure, equipment and its use.
Air Lock:
A compartment that has two or more doors separated between two or several rooms, such as between rooms of different cleanliness levels. The purpose of setting up the air lock is to control the airflow when the person or material enters and leaves. There is a lock between the air lock and the gas lock between the points.
The basic characteristics of biopharmaceutical cleansing plants are that they must be controlled by dust particles and microbes for the environment.
Drug production workshop cleanliness is divided into four levels: 100 or 10000 level of the local 100, 1000, 10000 and 30,000.
Clean room temperature: no special requirements, at 18 to 26 degrees, relative humidity control in 45% to 65%.
Biopharmaceutical Clean Plant Pollution Control: Pollution Source Control, Spreading Process Control, Cross Pollution Control.
Pharmaceutical plant clean room key technology is mainly to control dust and microbes, as a pollutant, microbial is the pharmaceutical plant clean room environmental control of the most important. Pharmaceutical plant clean area equipment, pipeline accumulation of pollutants, can directly pollute the drug, but without affecting the cleanliness of the test, so we said: GMP need air purification technology, and air purification technology does not mean GMP! The cleanliness level is not suitable for characterizing the physical, chemical, radioactive and vitality of suspended particles. Not familiar with the pharmaceutical production process and process, do not understand the causes of pollution and the accumulation of pollutants in the place, do not grasp the method of removing pollutants and evaluation criteria.
GMP technical transformation of the pharmaceutical plant project prevalence of the following:
Because of the existence of the subjective understanding of the errors in the pollution control process of the application of clean technology adverse, and ultimately appeared in some pharmaceutical companies invested heavily in transformation, the quality of drugs has not improved significantly.
The design and construction of pharmaceutical clean production plant, the equipment manufacturing, installation and production of raw and auxiliary materials, the quality of packaging materials, the implementation of the net equipment control procedures will affect the implementation of adverse product quality.
Construction of the impact of product quality is due to process control links in the process of installation and construction of hidden risks, the following specific performance:
① clean air conditioning system duct is not clean, the connection is not tight, air leakage rate is too large;
② color steel plate enclosure structure is not tight, clean room and technology sandwich (ceiling) of the improper sealing measures, closed doors do not close;
③ decorative profiles and process pipelines in the clean room to form a dead, dust;
④ individual location is not in accordance with the design requirements of construction, can not meet the relevant requirements;
⑤ the quality of the sealant used, but off, easy to fall off, deterioration;
⑥ back, exhaust color steel plate lane connected, dust from the exhaust to enter the air duct;
⑦ process purified water, water injection and other stainless steel sanitary pipe welding when the wall weld is not formed;
⑧ air duct check valve failure, air pollution caused by pollution;
⑨ drainage system installation quality, but off, pipe rack, accessories easy to dust;
⑩ clean room pressure adjustment failed, failed to meet the production process requirements.
GMP to find drug packaging workshop purification project environmental control requirements:
1, to provide the required level of production of air purification, packaging workshop purification works within the number of dust particles and live microorganisms should be regularly detected and recorded, different levels of packaging between the static pressure difference between the workshop should be maintained within the specified value;
2, packaging workshop purification project temperature and relative humidity should be compatible with its production process requirements;
3, penicillins, highly allergenic and anti-tumor drugs in the production area should be set up an independent air conditioning system, exhaust to clean up;
4, for the dusty room should be set up an effective dust collector to prevent dust pollution;
5, the storage and other auxiliary production room, its ventilation facilities and temperature and humidity should be compatible with the requirements of pharmaceutical production and packaging.
Second, to find drug packaging workshop purification project> cleanliness of the partition and the number of ventilation: clean room should strictly control the air cleanliness, and environmental temperature, humidity, fresh air and pressure and other parameters.
1, the pharmaceutical production and packaging workshop purification level and the number of ventilation and pharmaceutical production and packaging workshop purification works air cleanliness is divided into 100,
1 million, 100,000, 300,000 4 level. Determine the number of clean room ventilation, the need for the air volume to compare, take the maximum. In practice, 100 times the number of ventilation 300 to 400 times / h, 10,000 to 25-35 times / h, 100,000 to 15-20 times / h.
2, drug packaging workshop purification project cleanliness of the district drug production and packaging environment on the specific degree of cleanliness by the national standard cleanliness standards.
3, packaging workshop purification project to determine the other environmental parameters
4, packaging workshop purification project temperature and humidity clean room temperature and relative humidity should be consistent with the pharmaceutical production process.
Temperature: 100 and 10,000 to take 20 ~ 23 ~ C (summer),
100,000 and 300,000 to take 24 ~ 26 ~ C, the general area of 26 ~ 27 ~ C.
100 and 10,000 are sterile rooms. Relative humidity: easy to absorb moisture drugs 45% to 50% (summer), tablets and other solid preparations 50% to 55%, water and oral liquid 55% to 65%.
5, clean room pressure to maintain indoor cleanliness to maintain the indoor positive pressure. For the production of dust, harmful substances, the production of penicillin-like strong allergenic drugs such as the production of clean room to prevent external pollution or between the region to maintain a relatively negative pressure. Cleanliness level of inflow of static pressure in different rooms and outflow of internal air. Indoor to maintain a positive pressure, and adjacent rooms worse than 5Pa, clean room and outdoor air pressure difference is greater than 10Pa.
Dongguan Zhuo for the air-conditioning Electrical and Mechanical Equipment Co., Ltd. is specialized in the Pearl River Delta region clean clean workshop, the central air-conditioning engineering design and construction services of professional purification engineering company. Zhuo for purification in the electronics manufacturing, bio-pharmaceutical, printing and packaging, food, chemical optics, health care and other industries in the design and construction of numerous classic works, access to a high degree of customer recognition.